Pat 4 Of The 1983 Application
To Use Aspartame in Carbonated Beverages
submitted By A.G. Searle to the FDA
and reporting R&D done in 1973
Monte's reference link m48 gives all four sections of the petition and adds a cover page not related to the petition that is a little confusing to readers at first. The cover page is the note Dr. Monte received when he went to the FDA to review all documents concerning aspartame.
The summary first section of the petition says, "In chronic studies in normal adults and children, diabetics, and PKU heterozygote adults, 1.8 gm/day of aspartame were consumed for 13-21 weeks with no evidence of adverse effects." (That's the amount of aspartame in 10 cans/day of diet soda.)
Here's an outline of Monte's review of this report from pp.181-182 of his book.
|
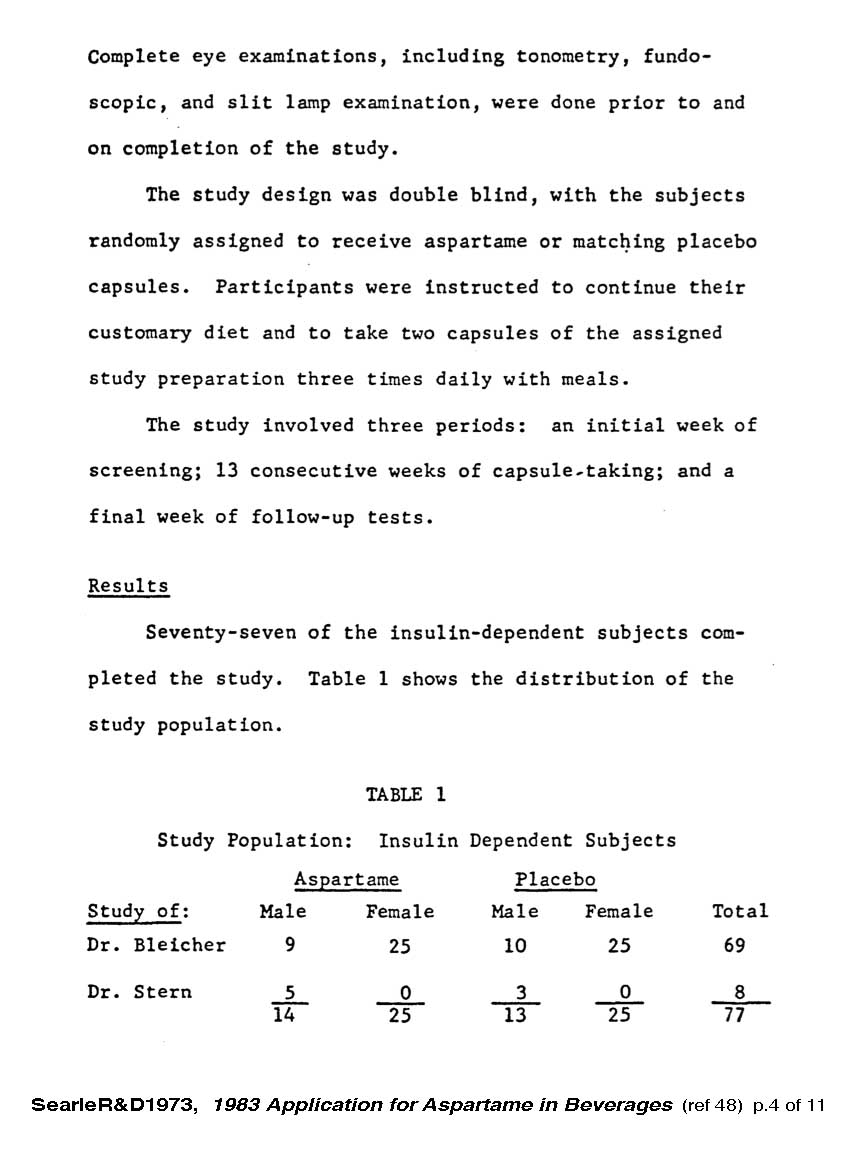
The tests in 1972-73 were to be of 13 weeks duration with 77 diabetic women who had been pre-screened to have no initial health problems. It was a double-blind study with half receiving the test substance, 1.8 g/day of aspartame. Three women in the aspartame group developed health problems and had to withdraw from the study - one because of fatal stroke at 8 weeks, and two because of epithelial cell cancers at 11 weeks.
Their type of hyperplasia and breast adenocarcinoma had been found in laboratory rats fed methanol during 1972 Searle testing released in 1977 (Bresslerm197), but those animal results were withheld from the pathologist Dr. Wagner in 1973 on p. 9-11 of this part 4 of the submission to the FDA. The pathologist was provided animal tissue slides by Searle and submitted his report concerning this petition directly to Searle. |
Here's the text of just Part 4 of the 1983 application: